Find Percent Acid in Buffer Solution Problem

Buffer preparation is a common process in chemistry and biochemistry laboratories. A buffer solution is a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffer solutions are used to help maintain a stable pH value of another solution that is mixed with the buffer. The buffer resists changes in the pH value of the whole solution when a small amount of a different acid or alkali is introduced into the solution either through addition or via any chemical reaction within the solution. Buffer solutions are therefore very useful in a wide variety of applications in which a relatively stable pH is required. A buffer may also be called a pH buffer, hydrogen ion buffer, or buffer solution.
For example, blood contains natural buffers to maintain a stable pH of between 7.35 and 7.45 so that our enzymes work correctly. As enzyme activity varies with pH, maintaining a constant pH is essential in biochemical assays to ensure the correct level of activity is observed. In commercial applications, buffers can be found in shampoos to prevent skin irritation, in baby lotions to inhibit the growth of bacteria, and in contact lens solutions to ensure the pH level of the fluid remains compatible with that of the eye.
Preparing buffers consists of several steps: Weighing-in the components, dissolving the components, adjusting the pH and replenishing to the final volume. As the ratio of the acid to base in a buffer is directly related to the final pH, it is vital to weigh the components with a high degree of accuracy. So, it is important that the equipment used (balance, pipettes and pH meter) are properly calibrated and have sufficient accuracy.
Watch the Video: Buffer Solution Preparation – Simple, Convenient and Accurate
Buffer solution preparation takes time and must be done with care to ensure the buffer performs as desired. When the quality of your products or your biochemical analyses depend on the performance of your buffer solutions, you want to be sure you get them right first time.
Watch the video and see how you can save time and effort preparing accurate buffer solutions with METTLER TOLEDO precision balances and pH meters.
Jump to one of the following section to explore and learn more:
- How to prepare buffer solutions? Typical procedure.
- Buffer solution know-how
- How do buffer solutions work?
- Types of buffer solutions
- What to consider when preparing a buffer solution
- Benefits of universal buffers
- Additional tips for preparing and using buffers
- Request free support for your buffer preparation process
- The challenges of preparing a buffer solution
- METTLER TOLEDO featured solution for convenient and accurate buffer preparation
- Weighing guide: Efficient weighing workflows
- Buffer preparation – an optimized workflow with comprehensive data management
- How to ensure your pH meter is calibrated correctly
- FAQ – frequently asked questions on buffer solution preparation
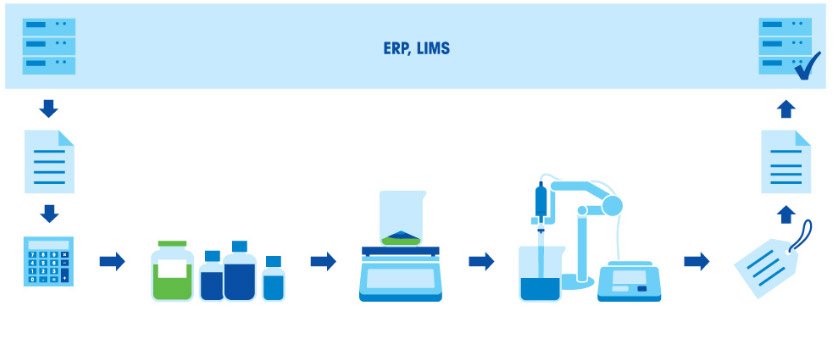
How to Prepare Buffer Solutions? Typical Procedure.
Preparing buffers consists of several steps: Calculate the components (concentrations and amounts) according to the required use and target volume, weigh-in the components, dissolve the components, adjust the pH, fill-up to the final volume, label, document results and use directly or store for later usage.

Do You Need Support?
Buffer solutions are vitally important in a wide range of applications. However, buffer preparation is time-consuming and labor-intensive and must be done with care to avoid errors. If you need support with buffer preparation or want advice on which balance meets your accuracy requirements, our team of experts is here for you. Do not hesitate to contact us for help or advice.

The Challenges of Preparing a Buffer Solution
Whilst the simplest buffer solution may consist just of an acid, a base and water, laboratory technicians often have to prepare several buffer solutions per day – typically consisting of 2-5 components, but could be as many as 20. A typical laboratory may have more than 20 buffer solution recipes that are intended to produce 1 liter of buffer.
Buffer solution calculations
With a database of 20+ recipes, the laboratory technician must first be sure that the correct buffer solution recipe is selected. For buffer solution volumes other than 1 liter, it is necessary to recalculate the quantities of all the components accordingly and log the new amounts. Any errors in the calculations or in recording the new values may lead to incorrect pH values in the buffer solution. There is a higher risk of errors in a manual data recording system.
Weighing and logging weight results
When weighing in all the different buffer solution components, care must be taken to use the right amount of the right component. The actual weight of each component must be logged, and, if recording results by hand or entering the values into a computer, care must be taken to avoid transcription errors.
Depending on how much of each of the buffer solution components is required and the minimum weight of the balance, it may be necessary to use two different balances. This complicates the process and reduces efficiency.
Correct performance of pH meters
Checking the pH level of the buffer solution is of vital importance. However, if the pH meter used is not calibrated and maintained correctly, pH readings may be inaccurate. Using a buffer solution with an incorrect pH may seriously impact subsequent analyses or product quality.
Documentation
The final buffer solution must be carefully labelled with all information to avoid any mix-ups. The expiry date is important to ensure the buffer solution is still effective when it is used. All data relating to the buffer solution preparation must be logged and stored securely for future reference and traceability.

METTLER TOLEDO Featured Solution for Convenient and Accurate Buffer Preparation
METTLER TOLEDO's XSR precision balances simplify buffer solution preparation and significantly reduce the workload for laboratory technicians.
Saved recipes
Up to 50 buffer solution recipes can be saved directly on the balance as weighing methods. By saving the most commonly used buffer solution recipes on the balance, it is quick to retrieve the method needed and start working. The balance provides step-by-step instructions so analysts can easily keep track of where they are in the buffer preparation process.
Automatic calculations and weighing-in guide
At the start of each method, the required volume of the buffer solution is entered and the component quantities are calculated automatically. As each ingredient is weighed in, the SmartTrac weighing guide displays the actual weight against the target weight graphically. The colored bar turns green as soon as the weight of the added component is within the predefined tolerance range. This enables analysts to weigh in more quickly and with greater certainty.
Efficient processes and automatic data recording
After each component has been added, the balance saves the actual weight result and then tares automatically, assisting in the weighing process and eliminating the need to manually record the results. The balance can be set to print out the weight results automatically once all the ingredients have been added.
Convenient pH measurement
METTLER TOLEDO's SevenExcellence pH meters are the perfect choice for accurate pH measurements. These easy-to-use instruments offer touchscreen operation, direct measurements at the touch of a button, and intelligent sensor management.
pH measurements are only as accurate as the calibration solutions used. METTLER TOLEDO also offers a selection of quality pH calibration solutions to match your specific requirements.

Guide: Biological Buffer Preparation
Discover how you can make your buffer preparation process easy and reliable.
Download the guide and get useful tips and hints to help improve the productivity of your preparation process, ensure quality standards and traceability for buffer solutions.
- Step-by-step SOP user guidance and accessible buffer recipes directly on the balance screen improves workflows
- Accurate weighing in compounds is vital for the quality of buffer solutions
- Exact and reliable pH measurement is important for the correct preparation of buffers
- Using and operating pipettes the correct way helps to ensure that pH buffer is properly adjusted
- Automatic calculation, labeling and reporting ensure safe data transfer and data traceability

Buffer Preparation – An Optimized Workflow with Comprehensive Data Management
METTLER TOLEDO Excellence Line precision balances (XPR and XSR) and SevenExcellence pH meters can be connected to our proprietary LabX laboratory software to create a fully optimized system that provides a range of key benefits:
- Automatic calculations and documentation
- All results saved in a secure database
- Full traceability
- Central management of tasks, users and instruments
Support with FDA 21CFR part 11 compliance
LabX provides comprehensive data management functions, practically eliminating manual data transcription – even detailed labels can be printed automatically. With a centralized database, all connected users and instruments can access the same stored SOPs for buffer preparation. This ensures that every analyst carries out buffer preparation with identical settings and parameters. All results and process metadata are saved automatically in a secure database so full traceability is assured.

How to Ensure Your pH Meter is Calibrated Correctly
To accurately calibrate your pH meter, you need accurate calibration buffer solutions of known pH! METTLER TOLEDO offers a range of quality pH buffers and guarantees maximum precision with NIST/DIN buffers.
Each buffer is supplied with a test certificate to help you ensure compliance and traceability. Whether you just need a technical buffer or a buffer certified by an accredited body, we have the right buffers to meet your needs.
Find Percent Acid in Buffer Solution Problem
Source: https://www.mt.com/hk/en/home/applications/Laboratory_weighing/buffer-preparation.html